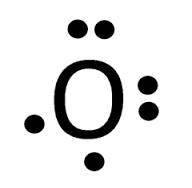
From the two lewis dot structures given, determine the formula for their ionic compound.
O2K
<...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
Questions

Mathematics, 22.10.2020 02:01

Biology, 22.10.2020 02:01



Mathematics, 22.10.2020 02:01

Chemistry, 22.10.2020 02:01


Chemistry, 22.10.2020 02:01


Biology, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

Computers and Technology, 22.10.2020 02:01


Geography, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01