
Chemistry, 09.02.2021 21:50 sweetyus06
Metals lose electrons in the formation of ionic bonds because they have relatively few valence electrons. Is it True or false
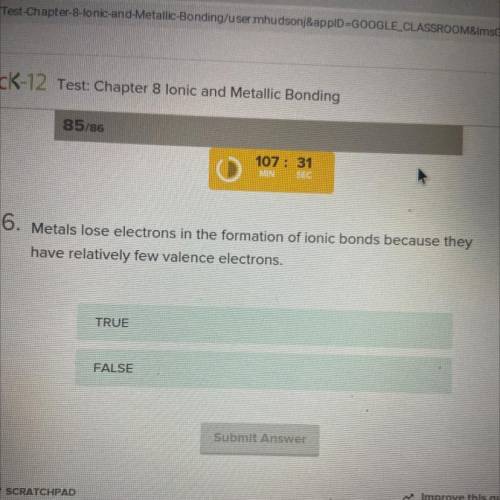

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Metals lose electrons in the formation of ionic bonds because they have relatively few valence elect...
Questions

Mathematics, 24.03.2021 19:30


Mathematics, 24.03.2021 19:30


Mathematics, 24.03.2021 19:30




Mathematics, 24.03.2021 19:30


Mathematics, 24.03.2021 19:30


Mathematics, 24.03.2021 19:30

Mathematics, 24.03.2021 19:30


Computers and Technology, 24.03.2021 19:30


Chemistry, 24.03.2021 19:30






