
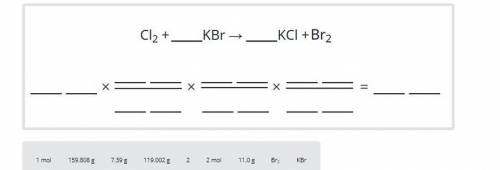
Balance the chemical equation. Based on the equation, how many grams of bromine are produced by the complete reaction of 11 grams of potassium bromide? Use the periodic table to get the weights of the elements. Carry out your calcuation in the same way as you did in parts A, B, and C. That is, begin by converting grams of KBr to moles of KBr, then use the mole ratio of KBr to Br2. Drag the labels to the correct locations to complete the analysis. Each label can be used more than once.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2



Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3
You know the right answer?
Balance the chemical equation. Based on the equation, how many grams of bromine are produced by the...
Questions



Geography, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

Business, 23.09.2020 14:01

Social Studies, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01




Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01





Spanish, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01



