
Chemistry, 27.01.2020 09:31 blakeolson0800
Achemist lectures about the following definition for acids and bases. the acid-base behavior is analyzed in terms of how electrons are transferred between compounds rather than in terms of how hydrogen ions are transferred. what is the chemist defining? the arrhenius concept of acids and bases the classical concept of acids and bases the lewis concept of acids and bases the bronsted-lowry concept of acids and bases

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Achemist lectures about the following definition for acids and bases. the acid-base behavior is anal...
Questions

Mathematics, 11.07.2019 01:30

Advanced Placement (AP), 11.07.2019 01:30

History, 11.07.2019 01:30

Health, 11.07.2019 01:30

History, 11.07.2019 01:30

Mathematics, 11.07.2019 01:30




English, 11.07.2019 01:30

Spanish, 11.07.2019 01:30


Mathematics, 11.07.2019 01:30


Mathematics, 11.07.2019 01:30

Computers and Technology, 11.07.2019 01:30


Mathematics, 11.07.2019 01:30

Mathematics, 11.07.2019 01:30

Mathematics, 11.07.2019 01:30

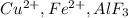 etcBases are electron pair donating species and they will have lone pairs of electrons. for example :
etcBases are electron pair donating species and they will have lone pairs of electrons. for example : 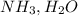 etc
etc

