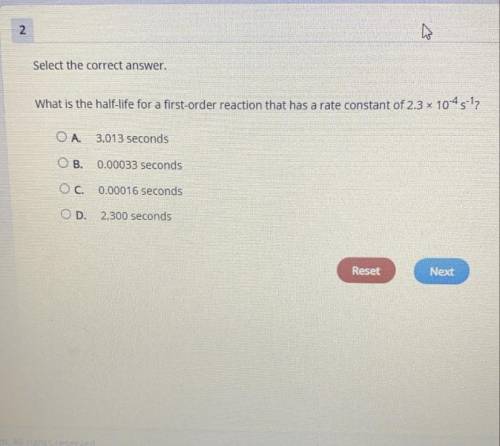
What is the half-life for a first-order reaction that has a rate constant of 2.3x10^-4 s^-1
...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

You know the right answer?
Questions



Chemistry, 15.12.2020 20:40

History, 15.12.2020 20:40



Mathematics, 15.12.2020 20:40

English, 15.12.2020 20:40


History, 15.12.2020 20:40





Mathematics, 15.12.2020 20:40

Arts, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

History, 15.12.2020 20:40

Health, 15.12.2020 20:40

English, 15.12.2020 20:40