Help me pleaseee
C3H8 (g) + 5 O2 (g)
->
3 CO2 (g) + 4 H2O (9)
1. Use sto...

Chemistry, 10.02.2021 20:40 natalie2sheffield
Help me pleaseee
C3H8 (g) + 5 O2 (g)
->
3 CO2 (g) + 4 H2O (9)
1. Use stoichiometry to determine how many moles of O2 are needed
to completely react with 2.25 moles of C3H8.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
Questions



Mathematics, 19.10.2020 22:01




Mathematics, 19.10.2020 22:01

Biology, 19.10.2020 22:01


Mathematics, 19.10.2020 22:01



English, 19.10.2020 22:01

History, 19.10.2020 22:01





Mathematics, 19.10.2020 22:01

Biology, 19.10.2020 22:01

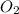 will be required to completely react with 2.25 moles of
will be required to completely react with 2.25 moles of 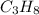 .
.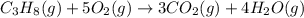
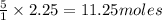 of
of 

