
Chemistry, 10.02.2021 22:20 gracethegreat1
Identify both of the PE wells in the graph below as belonging to either a covalent bond or an LDF. Then, explain what this means regarding the energy needed to break a covalent bond.
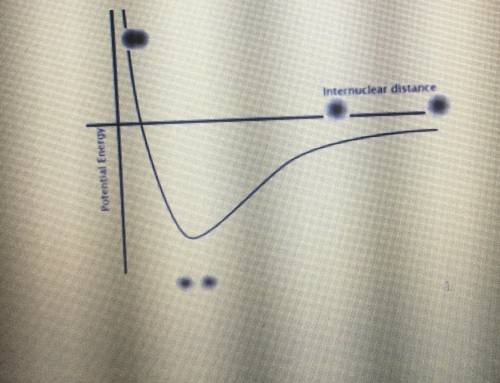

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Identify both of the PE wells in the graph below as belonging to either a covalent bond or an LDF. T...
Questions




History, 28.06.2019 08:30






Physics, 28.06.2019 08:30


Mathematics, 28.06.2019 08:30


Computers and Technology, 28.06.2019 08:30


History, 28.06.2019 08:30



History, 28.06.2019 08:30



