Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
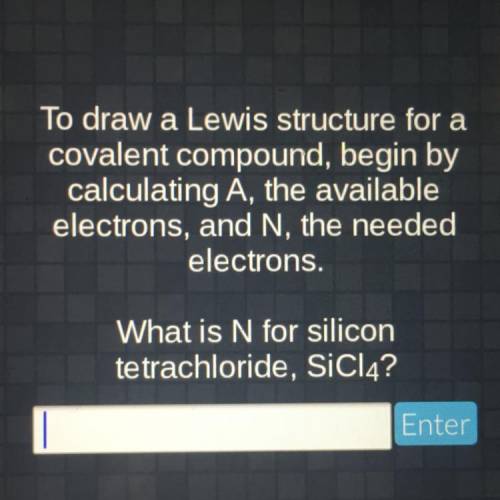
To draw a Lewis structure for a covalent compound, begin by calculating A, the available electrons,...
Questions


Mathematics, 07.04.2021 18:30




Mathematics, 07.04.2021 18:30

English, 07.04.2021 18:30

History, 07.04.2021 18:30

Arts, 07.04.2021 18:30

Mathematics, 07.04.2021 18:30

Mathematics, 07.04.2021 18:30

English, 07.04.2021 18:30

Social Studies, 07.04.2021 18:30



Mathematics, 07.04.2021 18:30

Mathematics, 07.04.2021 18:30

Computers and Technology, 07.04.2021 18:30


Mathematics, 07.04.2021 18:30