Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
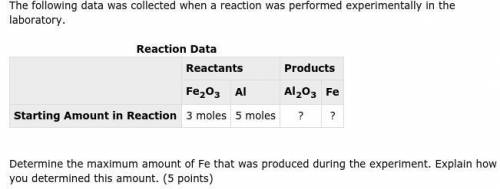
Please help! I have the balanced equation, Fe2O3+2Al -> Al2O3 + 2Fe
but I don't know where to go...
Questions


Social Studies, 31.07.2021 20:10

Chemistry, 31.07.2021 20:10



Social Studies, 31.07.2021 20:10

Physics, 31.07.2021 20:10


English, 31.07.2021 20:10


Chemistry, 31.07.2021 20:10



Computers and Technology, 31.07.2021 20:10

Mathematics, 31.07.2021 20:10

Mathematics, 31.07.2021 20:10

Mathematics, 31.07.2021 20:10



Social Studies, 31.07.2021 20:10