
Chemistry, 11.02.2021 07:00 Mypasswordishotdog11
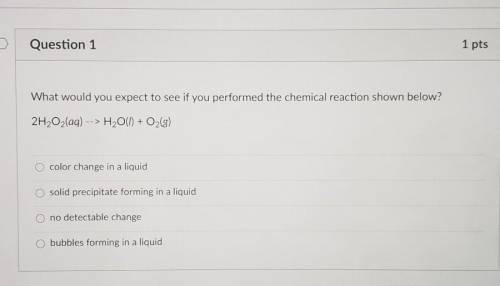
NEED THIS ASAP What would you expect to see if you performed the chemical reaction shown below? 2H2O2(aq) H2O(l) + O2(g) O color change in a liquid O solid precipitate forming in a liquid O no detectable change O bubbles forming in a liquid

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
NEED THIS ASAP
What would you expect to see if you performed the chemical reaction shown below? 2H2...
Questions





Mathematics, 18.07.2019 22:20





Medicine, 18.07.2019 22:20












