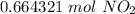Chemistry, 11.02.2021 08:30 pinkbutterfly03
Calculate the number of moles of nitrogen dioxide, that contain 4.0*1023 nitrogen dioxide molecule.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
Calculate the number of moles of nitrogen dioxide, that contain 4.0*1023 nitrogen dioxide molecule....
Questions

Computers and Technology, 18.10.2021 20:20



Mathematics, 18.10.2021 20:20

Health, 18.10.2021 20:20


Chemistry, 18.10.2021 20:20



Mathematics, 18.10.2021 20:20

Mathematics, 18.10.2021 20:20






History, 18.10.2021 20:20



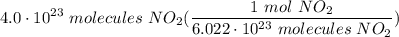 Multiply/Divide:
Multiply/Divide: