
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δh∘=−484kj
how much pv work is done in kilojoules for the reaction of 3.20 mol of h2 with 1.60 mol of o2 at atmospheric pressure if the volume change is −22.6l?
express your answer using three significant figures

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δ...
Questions



Mathematics, 25.05.2021 20:20


Mathematics, 25.05.2021 20:20

Mathematics, 25.05.2021 20:20



Mathematics, 25.05.2021 20:20

Mathematics, 25.05.2021 20:20



Mathematics, 25.05.2021 20:20

Mathematics, 25.05.2021 20:20

English, 25.05.2021 20:20

Mathematics, 25.05.2021 20:20


Mathematics, 25.05.2021 20:20


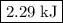 .
.
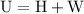
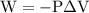
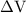 is the change in the volume in liter.
is the change in the volume in liter.
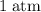 .
.
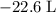 .
.
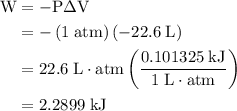
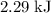 .
.


