
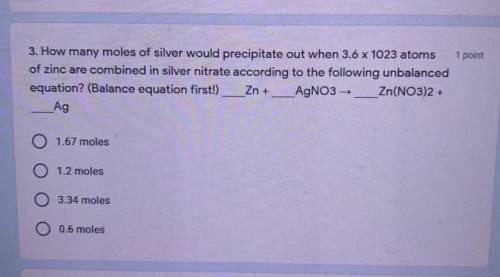
Chemistry, 11.02.2021 14:00 moomoofower
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver nitrate according to the following unbalanced equation? (Balance equation first!)
Zn + AgNO3 → Zn(NO3)2 +Ag
A: 1.67 moles
B: 1.2 moles
C: 3.34 moles
D: 0.6 moles
Can someone please help me with this I don’t get it at all.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1


Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver...
Questions

Mathematics, 31.07.2021 14:00

Mathematics, 31.07.2021 14:00

Mathematics, 31.07.2021 14:00


Social Studies, 31.07.2021 14:00


Physics, 31.07.2021 14:00

Mathematics, 31.07.2021 14:00


Physics, 31.07.2021 14:00

Business, 31.07.2021 14:00


Physics, 31.07.2021 14:00

English, 31.07.2021 14:00

Business, 31.07.2021 14:00

Biology, 31.07.2021 14:00

Biology, 31.07.2021 14:00

Mathematics, 31.07.2021 14:00

Social Studies, 31.07.2021 14:00

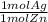 = 0.6 mol Ag
= 0.6 mol Ag

