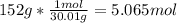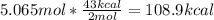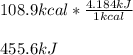Chemistry, 11.02.2021 14:00 santiagoagilg
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are produced in the following chemical reaction, 43
kcal of heat energy is "absorbed."
N2(g) + O2(g) → 2 NO(g), AH = +43 kcal
How much heat (in kJ) is exchanged when 152 g of NO(g) is produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are...
Questions


Mathematics, 16.04.2020 00:08

Mathematics, 16.04.2020 00:08

Mathematics, 16.04.2020 00:08

Physics, 16.04.2020 00:08




History, 16.04.2020 00:08

Mathematics, 16.04.2020 00:08


Mathematics, 16.04.2020 00:09

Mathematics, 16.04.2020 00:09

Mathematics, 16.04.2020 00:09

Mathematics, 16.04.2020 00:09

Mathematics, 16.04.2020 00:09




Mathematics, 16.04.2020 00:09