
Chemistry, 11.02.2021 14:00 abraham1366
Activity # 1: What's in the number?
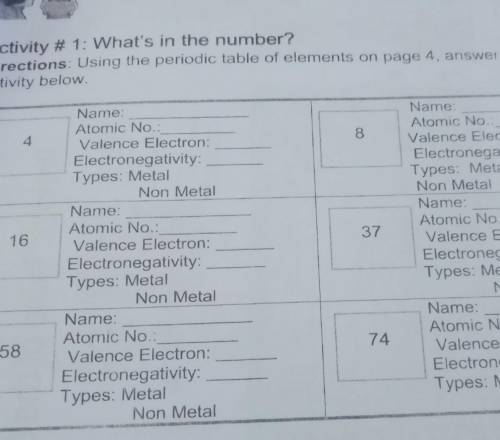
Directions: Using the periodic table of elements on page 4, answer the given
activity below
4
8
16
37
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
58
74 pa hellp plsss

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Activity # 1: What's in the number?
Directions: Using the periodic table of elements on page 4, ans...
Questions


Computers and Technology, 20.11.2019 01:31




















