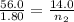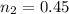Chemistry, 11.02.2021 17:20 hannahrasco4051
A sample of gas in a cylinder as in the example in Part A has an initial volume of 56.0 L , and you have determined that it contains 1.80 moles of gas. The next day you notice that some of the gas has leaked out. The pressure and temperature remain the same, but the volume has changed to 14.0 L . How many moles of gas (n2) remain in the cylinder

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
A sample of gas in a cylinder as in the example in Part A has an initial volume of 56.0 L , and you...
Questions





Chemistry, 05.05.2020 05:48





Biology, 05.05.2020 05:48

Mathematics, 05.05.2020 05:48

Geography, 05.05.2020 05:48

Social Studies, 05.05.2020 05:48

Chemistry, 05.05.2020 05:48

Biology, 05.05.2020 05:48


English, 05.05.2020 05:48

History, 05.05.2020 05:48


Chemistry, 05.05.2020 05:48

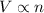
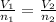
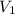 = initial volume of gas = 56.0 L
= initial volume of gas = 56.0 L
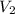 = final volume of gas = 14.0 L
= final volume of gas = 14.0 L
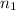 = initial moles of gas = 1.80
= initial moles of gas = 1.80 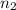 = final moles of gas = ?
= final moles of gas = ?