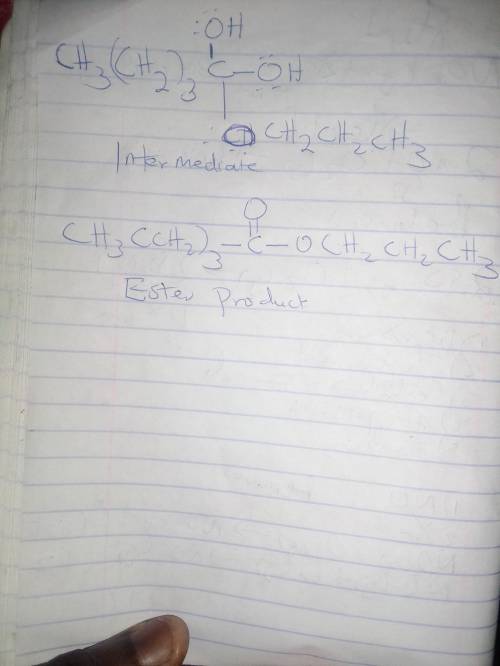Chemistry, 11.02.2021 18:00 unknown1246
In the Fischer esterification reaction, a carboxylic acid reacts with an excess of alcohol in acidic conditions to form an ester. During the reaction the sp2sp2 hybridized carbonyl carbon of the acid forms an sp3sp3 hybridized intermediate before returning to sp2sp2 hybridization in the product. Draw the structure of the neutral sp3sp3 hybridized intermediate and the ester product in the reaction between pentanoic acid and n‑propanol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 17:30
Alithium atom has three protons, three neutrons, and three electrons. what is the overall charge on this atom?
Answers: 1
You know the right answer?
In the Fischer esterification reaction, a carboxylic acid reacts with an excess of alcohol in acidic...
Questions

Mathematics, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00

History, 06.07.2019 02:00

Arts, 06.07.2019 02:00

History, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00

Arts, 06.07.2019 02:00

English, 06.07.2019 02:00




Mathematics, 06.07.2019 02:00

Biology, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00

Geography, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00

English, 06.07.2019 02:00