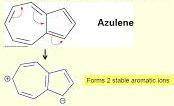
A resonance structure will be insignificant if it has carbon atoms with opposite charges (C- and C ). Azulene represents an exception to this rule, because some resonance structures (with C- and C ) exhibit aromatic stabilization. Draw a resonance structure of azulene with formal charges that best explains the aromatic stabilization. Include lone pairs in your answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1


Chemistry, 23.06.2019 14:30
When does phenolphthalein turn pink? in the presence of a base in the presence of an acid when it is in a neutral solution when it is reacting with a metal
Answers: 1
You know the right answer?
A resonance structure will be insignificant if it has carbon atoms with opposite charges (C- and C )...
Questions

Biology, 05.09.2020 19:01

Mathematics, 05.09.2020 19:01




Mathematics, 05.09.2020 19:01




Chemistry, 05.09.2020 19:01




Mathematics, 05.09.2020 19:01

Physics, 05.09.2020 19:01


History, 05.09.2020 19:01

Geography, 05.09.2020 19:01