
Chemistry, 11.02.2021 18:30 jcastronakaya
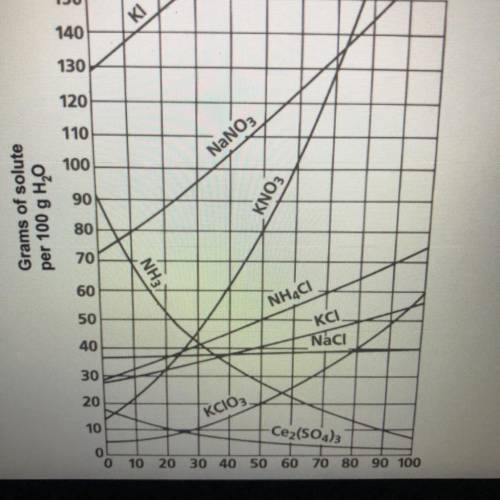
Continue to use the solubility curve graph to determine if the following solutions are
saturated or unsaturated. If they are unsaturated determine how much more solute
should be added to make a saturated solution.
7. (10 Points) A solution that contains 70g of NaNO3 at 30°C (in 100 mL H2O)
8. (10 Points) A solution that contains 50g of NH, Cl at 50°C (in 100 mL H,0)
9. (10 Points) A solution that contains 70g of KI at 0°C (in 100 mL H,0)
10. (10 Points) A solution that contains 20g of KCloz at 50°C (in 100 mL H2O)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Continue to use the solubility curve graph to determine if the following solutions are
saturated or...
Questions

Computers and Technology, 13.12.2020 09:30

Chemistry, 13.12.2020 09:30

Mathematics, 13.12.2020 09:30



Chemistry, 13.12.2020 09:30

Arts, 13.12.2020 09:30






Computers and Technology, 13.12.2020 09:40

Health, 13.12.2020 09:40


Mathematics, 13.12.2020 09:40

Mathematics, 13.12.2020 09:40


Medicine, 13.12.2020 09:40




