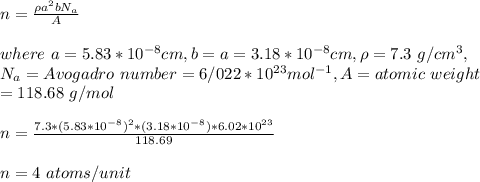Chemistry, 11.02.2021 21:10 yejinschoi6362
The unit cell for tin (Sn) has tetragonal symmetry, with a and b lattice parameters of 0.583 and 0.318 nm, respectively. If its density, atomic weight, and atomic radius are 7.30 g/cm3, 118.69 g/mol, and 0.151 nm, respectively. Determine its atomic packing factor.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
The unit cell for tin (Sn) has tetragonal symmetry, with a and b lattice parameters of 0.583 and 0.3...
Questions


Chemistry, 09.06.2021 22:00




Chemistry, 09.06.2021 22:00

Chemistry, 09.06.2021 22:00


Mathematics, 09.06.2021 22:00



English, 09.06.2021 22:00

Mathematics, 09.06.2021 22:00


History, 09.06.2021 22:00

French, 09.06.2021 22:00

Mathematics, 09.06.2021 22:00


Mathematics, 09.06.2021 22:00

Mathematics, 09.06.2021 22:00