
Chemistry, 12.02.2021 04:50 destinystanley3794
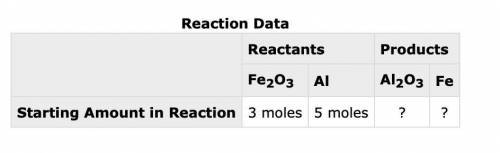
The following data was collected when a reaction was performed experimentally in the laboratory.
Reaction Data
Reactants Products
Fe2O3 Al Al2O3 Fe
Starting Amount in Reaction 3 moles 5 moles ? ?
Determine the maximum amount of Fe that was produced during the experiment. Explain how you determined this amount

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

You know the right answer?
The following data was collected when a reaction was performed experimentally in the laboratory.
Re...
Questions

Geography, 09.08.2019 19:20

English, 09.08.2019 19:20


Mathematics, 09.08.2019 19:20








Biology, 09.08.2019 19:20

Biology, 09.08.2019 19:20

Biology, 09.08.2019 19:20




Biology, 09.08.2019 19:20

Biology, 09.08.2019 19:20



