
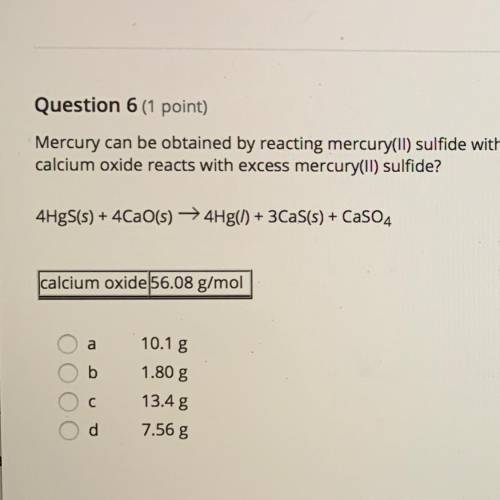
Chemistry, 12.02.2021 05:00 blakesmith0110
Mercury can be obtained by reacting mercury(II) sulfide with calcium oxide. How many grams of Mercury metal are produced when 2.11 g of calcium oxide reacts with excess mercury(II) sulfide?
4HgS+4CaO=4Hf+3CaS+CaSO4
a. 10.1 g
b. 1.80 g
c. 13.4 g
d. 7.56 g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1


Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1
You know the right answer?
Mercury can be obtained by reacting mercury(II) sulfide with calcium oxide. How many grams of Mercur...
Questions










History, 18.02.2020 21:00

Mathematics, 18.02.2020 21:00












