
Chemistry, 19.09.2019 18:30 woodfordmaliky
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(aq)+3h2(g) (deltah)= -725.0 kj
2. hcl(> hcl(aq) (deltah)= -74.8kj 3. h2(g)+cl2(g) --> 2hcl(g) (deltah)=-1845.0kj 4. mcl3(s) --> mcl3(aq) (deltah)= -476.0kj use the information above to determine the enthalpy of the following reaction.
2m(s)+3cl2(g) > 2mcl3(s) (deltah) = kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
Questions


Computers and Technology, 19.01.2021 19:20

Mathematics, 19.01.2021 19:20



Spanish, 19.01.2021 19:20

History, 19.01.2021 19:20




Arts, 19.01.2021 19:20



Mathematics, 19.01.2021 19:20



Physics, 19.01.2021 19:20


Biology, 19.01.2021 19:20

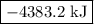
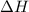 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.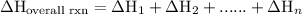
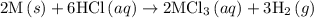 ...… (1)
...… (1)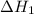 is -725 kJ.
is -725 kJ.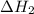 .
.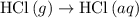 ...... (2)
...... (2) 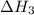 .
.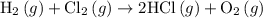 …… (3)
…… (3)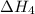 .
.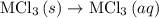 …… (4)
…… (4)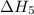 .
.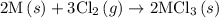 …… (5)
…… (5)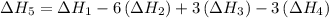 ...... (7)
...... (7) 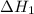 , -74.8 kJ for
, -74.8 kJ for 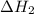 and -1845 kJ for
and -1845 kJ for 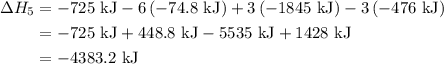
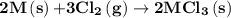 is -4383.2 kJ.
is -4383.2 kJ.

