
Chemistry, 12.02.2021 07:20 winterblackburn78
In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the gas constant, R, equal to . In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the gas constant, R, equal to . 8.314 m3-Pa/mol-K 0.08206 atm L mol-1K-1 1.987 cal mol-1K-1 62.36 L torr mol-1K-1 none of the above

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the...
Questions

Mathematics, 08.02.2021 16:10

Mathematics, 08.02.2021 16:10


Mathematics, 08.02.2021 16:10

English, 08.02.2021 16:10


History, 08.02.2021 16:10



Mathematics, 08.02.2021 16:10


History, 08.02.2021 16:10



Mathematics, 08.02.2021 16:10

SAT, 08.02.2021 16:10

Business, 08.02.2021 16:10

Mathematics, 08.02.2021 16:10


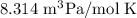
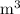
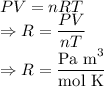
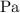 is used so the gas constant value that must be used is
is used so the gas constant value that must be used is 


