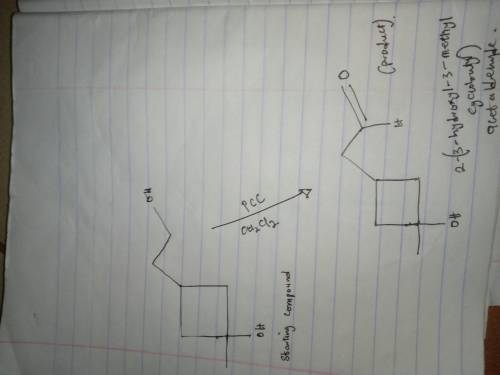
Give the product expected when the following alcohol reacts with pyridinium chlorochromate (PCC). (Assume that PCC is present in excess.) The starting material is a 4 carbon ring where carbon 1 is bonded to O H and C H 3. Carbon 3 is bonded to C H 2 C H 2 O H. This reacts with P C C in C H 2 C L 2 to give the product. Draw the product.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Give the product expected when the following alcohol reacts with pyridinium chlorochromate (PCC). (A...
Questions


Physics, 01.07.2020 17:01

Mathematics, 01.07.2020 17:01










Mathematics, 01.07.2020 17:01

Mathematics, 01.07.2020 17:01



Mathematics, 01.07.2020 17:01


Mathematics, 01.07.2020 17:01

Mathematics, 01.07.2020 17:01