
Chemistry, 12.02.2021 07:40 lillianbrowning10
CRQ QUESTION:
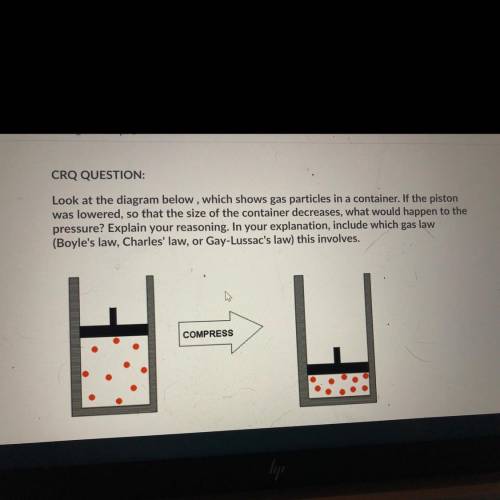
Look at the diagram below, which shows gas particles in a container. If the piston
was lowered, so that the size of the container decreases, what would happen to the
pressure? Explain your reasoning. In your explanation, include which gas law
(Boyle's law, Charles' law, or Gay-Lussac's law) this involves.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

You know the right answer?
CRQ QUESTION:
Look at the diagram below, which shows gas particles in a container. If the piston
Questions

Health, 20.10.2020 03:01

History, 20.10.2020 03:01










History, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01




Physics, 20.10.2020 03:01


Geography, 20.10.2020 03:01



