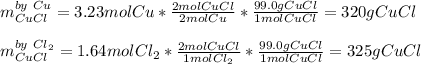Chemistry, 12.02.2021 14:00 Freindofafreind
2 Cu + Cl2 > 2 Cuci
If 1.64 moles of chlorine is reacted with 3.23 moles of copper, how many grams of copper I chloride will be made?
(The next question will ask about the limiting and excess reactants for this reaction)
o 320 g
O 160 g
O 162 g
O 325 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
2 Cu + Cl2 > 2 Cuci
If 1.64 moles of chlorine is reacted with 3.23 moles of copper, how many gra...
Questions


Mathematics, 16.10.2019 17:30


Chemistry, 16.10.2019 17:30





Chemistry, 16.10.2019 17:30







Chemistry, 16.10.2019 17:30


Social Studies, 16.10.2019 17:30