
Chemistry, 12.02.2021 14:10 alexis9263
Pete and Helen investigate the reaction between sodium carbonate, Na2CO3, and ethanoic acid, C2H4O2. Sodium ethanoate, C2H3O2Na, carbon dioxide and water are made. Pete and Helen measure the mass of carbon dioxide made every 30 seconds during the reaction. They do the experiment again. They use the same amount of acid and sodium carbonate. This time they use warm ethanoic acid instead of cold ethanoic acid. Look at the graph below. It shows their results. Look at the graph for the warm acid. How many minutes does it take for the reaction to finish?
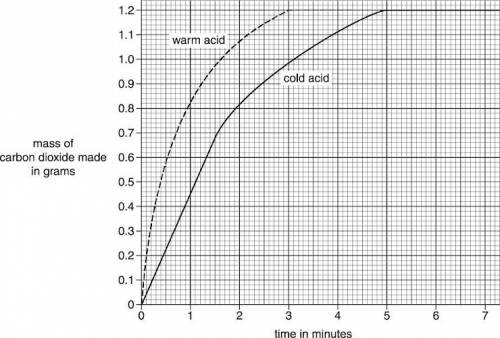

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Pete and Helen investigate the reaction between sodium carbonate, Na2CO3, and ethanoic acid, C2H4O2....
Questions


Social Studies, 17.09.2019 04:30


Social Studies, 17.09.2019 04:30

Physics, 17.09.2019 04:30

Mathematics, 17.09.2019 04:30

Mathematics, 17.09.2019 04:30

Chemistry, 17.09.2019 04:30


History, 17.09.2019 04:30

Physics, 17.09.2019 04:30

Spanish, 17.09.2019 04:30


Mathematics, 17.09.2019 04:30


Arts, 17.09.2019 04:30

Mathematics, 17.09.2019 04:30


Mathematics, 17.09.2019 04:30

Mathematics, 17.09.2019 04:30



