
Chemistry, 12.02.2021 21:30 dcttechgames
Can you show your work and answer these questions? will definitely give brainliest
Additional two parts:
f.) Calculate the change in entropy from the data below. Explain whether or not the data supports your answer above.
g.)Which species would be present in higher concentration at 298K --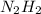 or
or 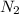 ? Justify your answer in terms of thermodynamic favorability and the equilibrium constant.
? Justify your answer in terms of thermodynamic favorability and the equilibrium constant.
![\left[\begin{array}{ccc}Chemical&Entropy(J/(mol*k))\\N_{2}H_{4}(g) &238.7\\H_{2}O_{2}(g)&232.6\\H_{2}O(g)&188.8\\N_{2}(g)&191.6\end{array}\right]](/tpl/images/2145/7103/19c01.png)



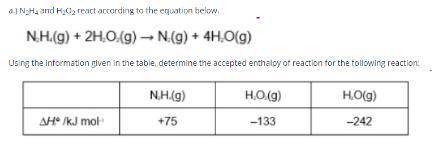


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Can you show your work and answer these questions? will definitely give brainliest
Additional two p...
Questions

Mathematics, 12.07.2019 22:20







Biology, 12.07.2019 22:20

Mathematics, 12.07.2019 22:20

Mathematics, 12.07.2019 22:20

Mathematics, 12.07.2019 22:20

English, 12.07.2019 22:20











