Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
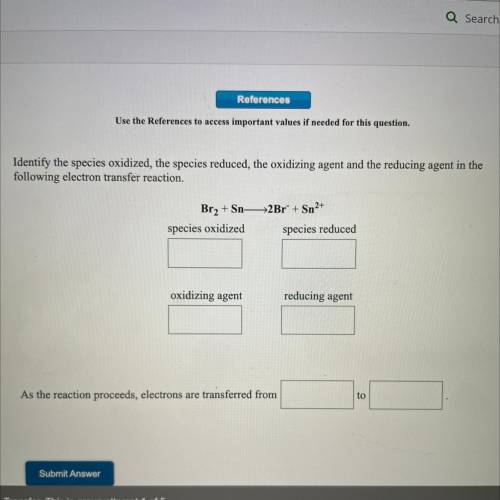
Br2 + Sn -> 2Br^- + Sn^2+
species oxidized:
species reduced:
oxidizing agent:
...
species reduced:
oxidizing agent:
...
Questions





Mathematics, 15.04.2020 17:24

Mathematics, 15.04.2020 17:24



Mathematics, 15.04.2020 17:24


Mathematics, 15.04.2020 17:24



English, 15.04.2020 17:24