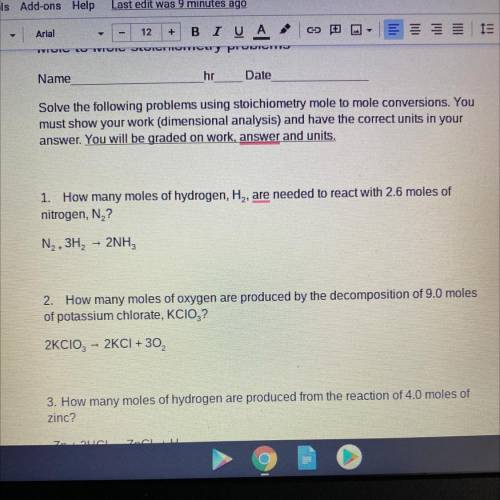
1. How many moles of hydrogen, H2, are needed to react with 2.6 moles of
nitrogen, N2?
N2-3H2...

Chemistry, 12.02.2021 21:40 ndkdidnfnk
1. How many moles of hydrogen, H2, are needed to react with 2.6 moles of
nitrogen, N2?
N2-3H2
-
2NH2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
Questions

Mathematics, 29.08.2019 21:00

Spanish, 29.08.2019 21:00

Chemistry, 29.08.2019 21:00

History, 29.08.2019 21:00

Physics, 29.08.2019 21:00

Geography, 29.08.2019 21:00

History, 29.08.2019 21:00

History, 29.08.2019 21:00

Mathematics, 29.08.2019 21:00


Mathematics, 29.08.2019 21:00


Social Studies, 29.08.2019 21:00

Social Studies, 29.08.2019 21:00

World Languages, 29.08.2019 21:00


Geography, 29.08.2019 21:00

English, 29.08.2019 21:00

Social Studies, 29.08.2019 21:00

History, 29.08.2019 21:00



