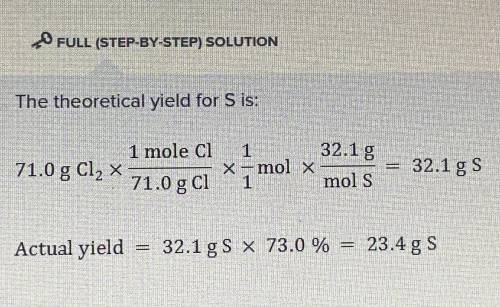
Consider the following reaction:
Cl2 + Na2S -> 2NaCl + S
The percent yield of sulfur...

Chemistry, 13.02.2021 04:20 yupthatsme2121
Consider the following reaction:
Cl2 + Na2S -> 2NaCl + S
The percent yield of sulfur when 71.0 g of Cl2 is reacted in excess Na2S solution is 73.0 %.
What is the actual mass of sulfur yielded by this reaction?
(Molar mass of S = 32.1 g/mol, molar mass of Cl2 = 71.0 g/mol)
__ g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2
You know the right answer?
Questions

Mathematics, 11.10.2021 01:30

Mathematics, 11.10.2021 01:30

Mathematics, 11.10.2021 01:30

English, 11.10.2021 01:30

Engineering, 11.10.2021 01:30

Mathematics, 11.10.2021 01:30



Mathematics, 11.10.2021 01:30


Mathematics, 11.10.2021 01:30

Mathematics, 11.10.2021 01:30



Mathematics, 11.10.2021 01:30

Social Studies, 11.10.2021 01:30

English, 11.10.2021 01:30


Mathematics, 11.10.2021 01:30

Advanced Placement (AP), 11.10.2021 01:30