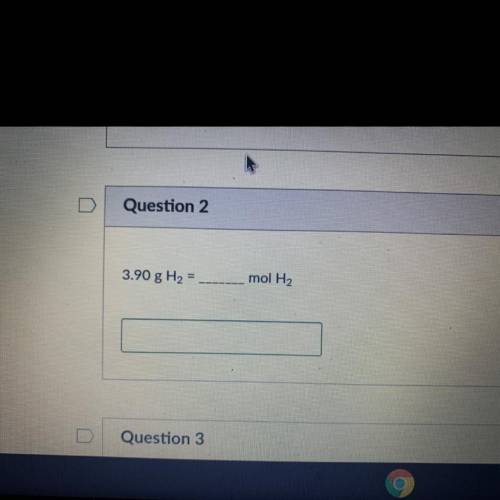
Help asap please i’m really struggling
...

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 08:00
Why is it important for scientists to review and repeat the work of other scientists? 1.a scientific theory must be tested three times before it is proven. 2.the scientific method only applies to repeated experiments. 3.an experiment may have had errors that the scientists didn't recognize. 4.the results of individual scientists may be influenced by bias. 5.an experiment must be performed twice before the data can be analyzed.
Answers: 3

Chemistry, 23.06.2019 13:30
How many ammonium ions and how many sulfate ions are present in a 0.270 mol sample of ?
Answers: 1
You know the right answer?
Questions

Mathematics, 11.03.2021 19:10

Mathematics, 11.03.2021 19:10



Mathematics, 11.03.2021 19:10

Mathematics, 11.03.2021 19:10

English, 11.03.2021 19:10





Mathematics, 11.03.2021 19:10


Social Studies, 11.03.2021 19:10

Biology, 11.03.2021 19:10

Mathematics, 11.03.2021 19:10


History, 11.03.2021 19:10