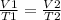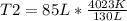Chemistry, 14.02.2021 06:20 annetteaudc
I have 130 liters of gas in a piston at a temperature of 3750 C. If I cool the gas until the volume decreases to 85 liters, what will temperature of the gas be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

You know the right answer?
I have 130 liters of gas in a piston at a temperature of 3750 C. If I cool the gas until the volume...
Questions


Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30


Biology, 15.12.2020 21:30




Mathematics, 15.12.2020 21:30



Mathematics, 15.12.2020 21:30






English, 15.12.2020 21:30