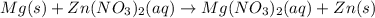Chemistry, 14.02.2021 14:00 kordejah348
What has a complete ionic equation of Mg(s) + Zn2+ + 2NO3 → Mg2+ + 2NO3 + Zn(s)?
O A. Mg(s) + Zn2+ → Mg2+ + Zn(s)
O B. Mg(s) + Zn(NO3)2(aq) + Mg(NO3)2(aq) + Zn(s)
O C. Mg(NO3)2 + Zn(s) → Zn2+ + 2NO3 + Mg(s) O D. Mg(s) + 2Zn(NO3)(aq) + 2MgNO3(aq) + Zn(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
What has a complete ionic equation of Mg(s) + Zn2+ + 2NO3 → Mg2+ + 2NO3 + Zn(s)?
O A. Mg(s) + Zn2+...
Questions

English, 29.09.2021 17:30

History, 29.09.2021 17:30


Mathematics, 29.09.2021 17:40



Mathematics, 29.09.2021 17:40

History, 29.09.2021 17:40

Mathematics, 29.09.2021 17:40

Mathematics, 29.09.2021 17:40

Mathematics, 29.09.2021 17:40

Mathematics, 29.09.2021 17:40


Mathematics, 29.09.2021 17:40



Business, 29.09.2021 17:40


Mathematics, 29.09.2021 17:40