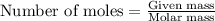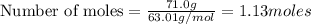Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
You know the right answer?
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles...
Questions

English, 01.07.2021 15:20

Mathematics, 01.07.2021 15:20




Social Studies, 01.07.2021 15:20












Computers and Technology, 01.07.2021 15:20


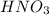 means 71.0 g of
means 71.0 g of