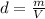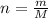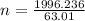Chemistry, 14.02.2021 14:00 patrick171888
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles of HNO3 are present in 1.98 L of this solution?
a.
62.8 mol
b.
31.7 mol
c.
22.3 mol
d.
0.0316 mol
e.
none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles...
Questions

Mathematics, 08.10.2021 20:50

History, 08.10.2021 20:50


History, 08.10.2021 20:50

Social Studies, 08.10.2021 20:50





English, 08.10.2021 20:50




Mathematics, 08.10.2021 20:50


Mathematics, 08.10.2021 20:50

Mathematics, 08.10.2021 20:50


History, 08.10.2021 20:50