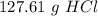Chemistry, 15.02.2021 04:10 gulleraliyeva1999
one reaction that produces hydrogen gas can be represented by the unbalanced chemical equation Mg(s)+HCI(aq) -> MgCI(aq)+H2(g). What is the mass of HCI is consumed by the reaction of 3.25 mol of magnesium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
one reaction that produces hydrogen gas can be represented by the unbalanced chemical equation Mg(s)...
Questions

Social Studies, 04.01.2020 03:31

Social Studies, 04.01.2020 03:31







Mathematics, 04.01.2020 03:31



Biology, 04.01.2020 03:31

History, 04.01.2020 03:31


World Languages, 04.01.2020 03:31

Biology, 04.01.2020 03:31

Chemistry, 04.01.2020 03:31

Business, 04.01.2020 03:31


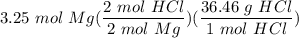 [S - DA] Multiply/Divide [Cancel out units]:
[S - DA] Multiply/Divide [Cancel out units]: