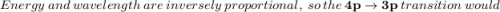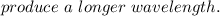Chemistry, 15.02.2021 19:40 raivynvieu6605
According to the quantum-mechanical model for the hydrogen atom, which electron transition produces light with the longer wavelength: 3p + 2s or 4p + 3p? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help increases As the n level increases, the energy and thus the levels are each 3p → 2s other. Therefore, the transition would have a greater energy difference than the transition from inversely decreases Energy and wavelength are proportional, so the transition would farther from produce a longer wavelength. 4p + 3p directly closer to

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1
You know the right answer?
According to the quantum-mechanical model for the hydrogen atom, which electron transition produces...
Questions





Spanish, 19.10.2020 14:01


Health, 19.10.2020 14:01