Chemistry, 15.02.2021 20:30 odalyarreola18
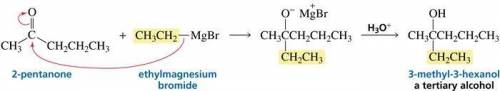
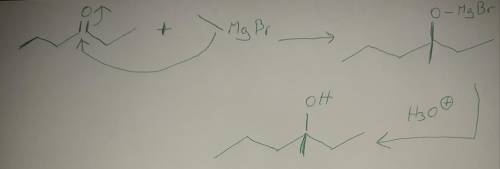
We see below that 3-methyl-3-hexanol can be synthesized from the reaction of 2-pentanone with ethylmagnesium bromide. There is a scheme of a chemical reaction, where CH3CCH2CH2CH3, with an O atom double-bonded to the second (from left to right) carbon (2-pentanone) reacts with CH3CH2MgBr (ethylmagnesium bromide). The arrow goes from the single bond between CH3CH2 and MgBr to the second C atom of 2-pentanone. The second arrow goes from the double bond between the second C atom and the O atom of 2-pentanone to the O atom. An intermediate is CH3CCH2CH2CH3, with a CH2CH3 group and an O minus MgBr plus group attached to the second carbon. In the presence of H3O plus the intermediate goes to CH3CCH2CH2CH3, with a CH2CH3 group and an OH group attached to the second carbon (3-methyl-3-hexanol, a tertiary alcohol). What other combinations of ketone and Grignard reagent could be used to prepare the same tertiary alcohol

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
We see below that 3-methyl-3-hexanol can be synthesized from the reaction of 2-pentanone with ethylm...
Questions

Social Studies, 28.08.2019 05:00



Mathematics, 28.08.2019 05:00


Chemistry, 28.08.2019 05:00




Mathematics, 28.08.2019 05:00



English, 28.08.2019 05:00

History, 28.08.2019 05:00

Mathematics, 28.08.2019 05:00



Biology, 28.08.2019 05:00

Mathematics, 28.08.2019 05:00

Health, 28.08.2019 05:00