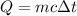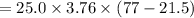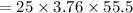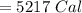Chemistry, 16.02.2021 01:00 brattymoo1009
The specific heat of a metal is 3.76 cal/g°C. How much heat energy will it take to heat a 25.0 g cylinder of the metal from 21.5°C to 77.0°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
The specific heat of a metal is 3.76 cal/g°C. How much heat energy will it take to heat a 25.0 g cyl...
Questions

Business, 22.12.2022 17:10

English, 22.12.2022 17:20

Mathematics, 22.12.2022 17:20


Mathematics, 22.12.2022 17:40

English, 22.12.2022 17:40




English, 22.12.2022 22:37

Social Studies, 23.12.2022 01:00




Computers and Technology, 23.12.2022 13:10


Mathematics, 23.12.2022 22:50

Mathematics, 24.12.2022 03:30