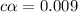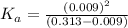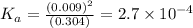Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

You know the right answer?
In the laboratory, a general chemistry student measured the pH of a 0.313 M aqueous solution of acet...
Questions

Mathematics, 25.02.2021 09:20

Social Studies, 25.02.2021 09:20



English, 25.02.2021 09:20

Mathematics, 25.02.2021 09:20


English, 25.02.2021 09:20

Computers and Technology, 25.02.2021 09:20

Mathematics, 25.02.2021 09:20


Physics, 25.02.2021 09:20


French, 25.02.2021 09:20




Social Studies, 25.02.2021 09:20


Mathematics, 25.02.2021 09:20

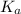 for the acid is
for the acid is 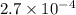
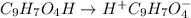
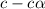
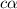
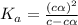
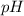 = 2.031
= 2.031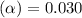
![[H^+]=c\times \alpha](/tpl/images/1121/0617/4fc41.png)
![[H^+]=0.500\times 0.030=0.015](/tpl/images/1121/0617/28636.png)
![pH=-log[H^+]](/tpl/images/1121/0617/15713.png)
![2.031=-log[H^+]](/tpl/images/1121/0617/0b1a6.png)
![[H^+]=0.009](/tpl/images/1121/0617/13241.png)
![[H^+]=c\alpha](/tpl/images/1121/0617/21a04.png)