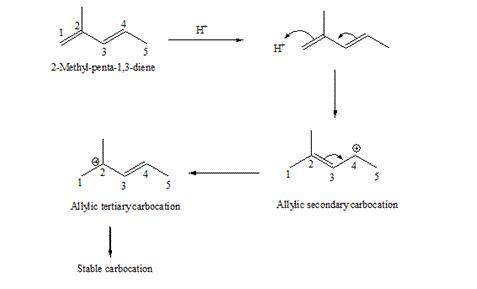
. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of 2-methyl-1,3-pentadiene. Without doing a calculation, would you expect C-2 or C-4 (the two end carbons of the allylic cation) to have the most positive charge on it

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of...
Questions

English, 04.10.2020 22:01


Mathematics, 04.10.2020 22:01




Mathematics, 04.10.2020 22:01

Mathematics, 04.10.2020 22:01

Geography, 04.10.2020 22:01

Mathematics, 04.10.2020 22:01



Mathematics, 04.10.2020 22:01





World Languages, 04.10.2020 22:01

History, 04.10.2020 22:01