
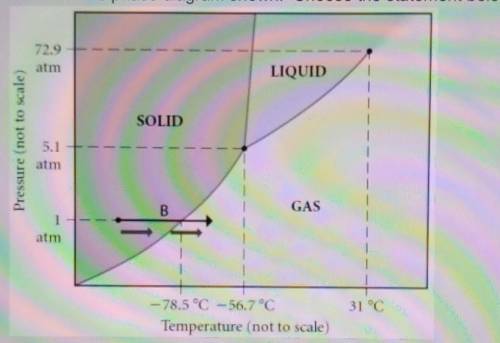
Select one: a. The line separating the solid and liquid phases represents the H vap b. The triple point of this substance occurs at a temperature of 31°C. c. The solid phase of this substance is higher in density than the liquid phase. d. At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. e. None of the above are true. which is true?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
Select one: a. The line separating the solid and liquid phases represents the H vap b. The triple po...
Questions

Mathematics, 16.02.2022 20:40


Social Studies, 16.02.2022 20:40


Mathematics, 16.02.2022 20:40

Mathematics, 16.02.2022 20:40

Social Studies, 16.02.2022 20:40







Geography, 16.02.2022 20:40


English, 16.02.2022 20:40


Mathematics, 16.02.2022 20:40

Mathematics, 16.02.2022 20:40



