Chemistry, 16.02.2021 04:20 baileyflemingde
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How many grams of sodium azide are required to produce 24.4 L of nitrogen gas at standard temperature and pressure? 2NaN3 --> 2Na + 3N2
47.2 g of sodium azide
106.2 g of sodium azide
1.63 g of sodium azide
0.726 g of sodium azide

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How...
Questions



Mathematics, 21.05.2020 07:02

Mathematics, 21.05.2020 07:02




Biology, 21.05.2020 07:02



History, 21.05.2020 07:02

Computers and Technology, 21.05.2020 07:02

Geography, 21.05.2020 07:02

English, 21.05.2020 07:02


Mathematics, 21.05.2020 07:02

Chemistry, 21.05.2020 07:02

English, 21.05.2020 07:02


Mathematics, 21.05.2020 07:02

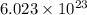 of particles.
of particles.
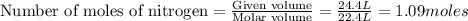
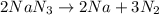
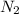 are produced by = 2 moles of
are produced by = 2 moles of 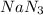
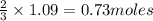 of
of