
Chemistry, 16.02.2021 18:50 screamqueen
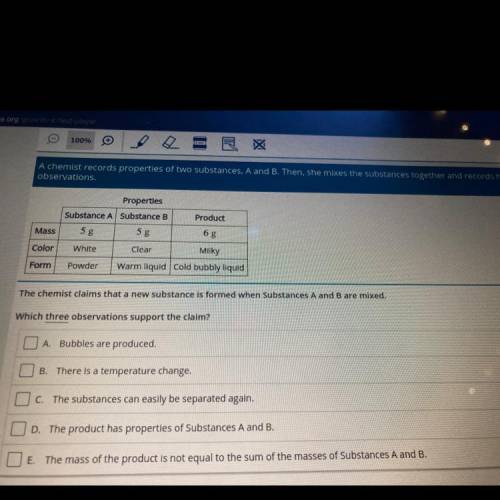
A chemist records properties of two substances, A and B. Then, she mixes the substances together and records her
observations.
Product
Properties
Substance A Substance B
5 g
5 g
White
Clear
Mass
Color
Milky
Form
Powder
Warm liquid cold bubbly liquid
The chemist claims that a new substance is formed when Substances A and B are mixed.
Which three observations support the claim?
O A Bubbles are produced.
B
There is a temperature change.
O c. The substances can easily be separated again.
OD. The product has properties of Substances A and B.
O E. The mass of the product is not equal to the sum of the masses of Substances A and B.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 01:30
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
A chemist records properties of two substances, A and B. Then, she mixes the substances together and...
Questions

Computers and Technology, 26.04.2021 21:40


Spanish, 26.04.2021 21:40


SAT, 26.04.2021 21:40


Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40



Mathematics, 26.04.2021 21:40




English, 26.04.2021 21:40



Mathematics, 26.04.2021 21:40



