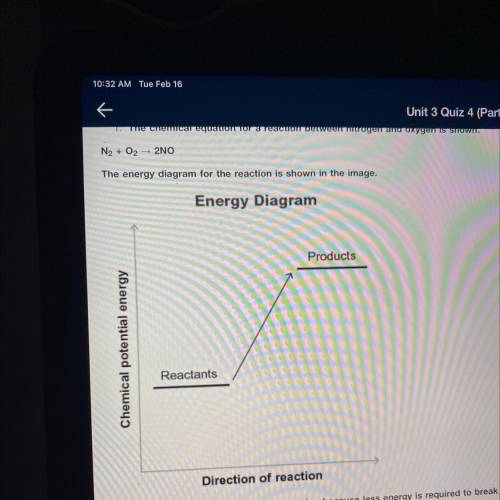
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
T...

Chemistry, 16.02.2021 21:50 brookie125
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
Then energy diagram for the reaction is shown in the image.
A) energy is absorbed, less energy, break bonds, form new bonds.
B) energy is released, more energy, break bonds, form new bonds
C) Energy is absorbed, the bond energy of the reactant is higher than the bond energy of the products
D) Energy is released, the bond energy of the reactant is lower than the bond energy of the products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Questions




Mathematics, 20.09.2020 14:01



Mathematics, 20.09.2020 14:01




Mathematics, 20.09.2020 14:01



Mathematics, 20.09.2020 14:01

Biology, 20.09.2020 14:01




Mathematics, 20.09.2020 14:01

Social Studies, 20.09.2020 14:01



