
Chemistry, 17.02.2021 01:00 maritzahernandez32
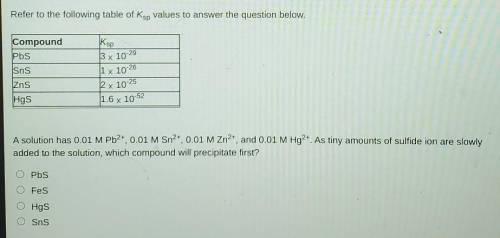
Refer to the following table of Ksp values to answer the question below. Compound
A solution has 0.01 M Pb2+, 0.01 M Sn2+, 0.01 M Zn2+, and 0.01 M Hg2+. As tiny amounts of sulfide ion are slowly added to the solution, which compound will precipitate first?
A. Pbs
B. Fes
C. Hgs
D. Sns

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Refer to the following table of Ksp values to answer the question below. Compound
A solution has 0...
Questions

Business, 25.02.2022 18:00


Mathematics, 25.02.2022 18:00


Mathematics, 25.02.2022 18:10


Arts, 25.02.2022 18:10

Mathematics, 25.02.2022 18:10


Mathematics, 25.02.2022 18:10

Biology, 25.02.2022 18:10




Mathematics, 25.02.2022 18:10


Computers and Technology, 25.02.2022 18:10



Advanced Placement (AP), 25.02.2022 18:10



