
Chemistry, 17.02.2021 01:10 gui00g7888888888888
For the reaction below, the order of reaction for NH4+ is 1, and the order of reaction for NO2 is 1.
NH4(aq) + NO3(aq) = N2(g) + 2H2O(1)
What is the rate law for the reaction?
A. R=k[NH+4] [NO-2]
B. R= [NH+4] [NO-2]2
C. R=k[NH]4 [NO]2
D. R=K[N2][H20]
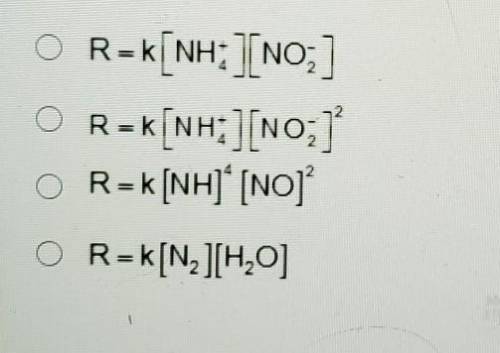

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
For the reaction below, the order of reaction for NH4+ is 1, and the order of reaction for NO2 is 1....
Questions

Business, 31.07.2019 14:10

Mathematics, 31.07.2019 14:10

History, 31.07.2019 14:10

Mathematics, 31.07.2019 14:10

Geography, 31.07.2019 14:10


Mathematics, 31.07.2019 14:10








Mathematics, 31.07.2019 14:10

English, 31.07.2019 14:10

Biology, 31.07.2019 14:10

Mathematics, 31.07.2019 14:10


Mathematics, 31.07.2019 14:10



