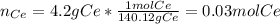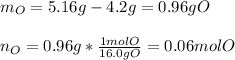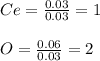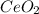Chemistry, 17.02.2021 08:50 Lizzyloves8910
4.2g of cerium reacted with oxygen to form 5.16g of an oxide of cerium. Find
the simplest formula of this oxide*
ASAP plz

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

You know the right answer?
4.2g of cerium reacted with oxygen to form 5.16g of an oxide of cerium. Find
the simplest formula o...
Questions

Social Studies, 28.01.2020 20:49

Mathematics, 28.01.2020 20:49


Mathematics, 28.01.2020 20:49

English, 28.01.2020 20:49



Chemistry, 28.01.2020 20:49

Mathematics, 28.01.2020 20:49






Mathematics, 28.01.2020 20:49





Mathematics, 28.01.2020 20:49